| One lithium (Li) atom can combine with one fluorine (F) atom. Together, they make the formula LiF. Fluorine has seven electrons of it's own. Lithium gives up its one electron to make both atoms happy. So the fluorine atom has eight electrons, and a filled outer shell. | ||
| Two fluorine (F) atoms can also bond with one beryllium (Be) atom, making the formula BeF2. Beryllium gives up one of it's electrons to each of the fluorine atoms. The result give each of the fluorines eight electrons, making their shells full. | ||
| Fluorine (F) can also bond with aluminum (Al). Aluminum has three extra electrons and will easily let the fluorine atoms use them. Because aluminum has three, that means three fluorines can bond. The make the formula AlF3, also known as aluminum trifluoride. Each of the fluorine atoms gets an electron to fill their shell, and the aluminum loses three, giving it a filled shell too (remember, aluminum has three extra electrons). The name trifluoride means three fluorine atoms are involved. | ||

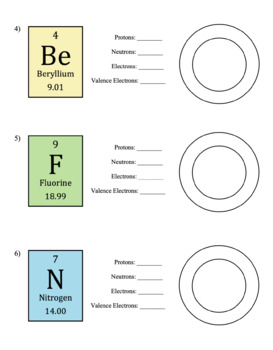
Fluorine Ion Protons Neutrons Electrons


Fluorine Protons Neutrons Electrons
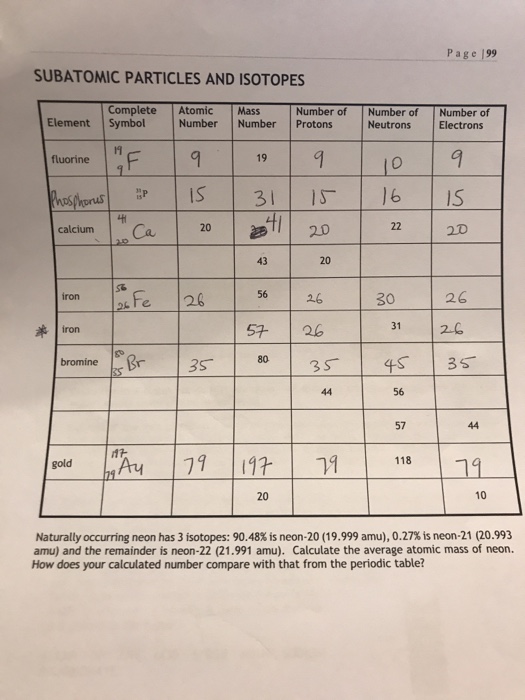
So for the element of FLUORINE, you already know that the atomic number tells you the number of electrons. That means there are 9 electrons in a fluorine atom. Looking at the picture, you can see there are two electrons in shell one and seven in shell two. What is the number of neutrons in fluorine? One lithium (Li) atom can combine with one fluorine (F) atom. Together, they make the formula LiF. Fluorine has seven electrons of it's own. Lithium gives up its one electron to make both atoms happy. So the fluorine atom has eight electrons, and a filled outer shell. Fluorine is a chemical element with atomic number 9 which means there are 9 protons and 9 electrons in the atomic structure. The chemical symbol for Fluorine is F. Neutron Number and Mass Number of Fluorine Mass numbers of typical isotopes of Fluorine are 19.
